Abstract
Introduction: Epigenetic regulatory mechanisms such as the modification of histone residues are perturbed in myeloid neoplasms. Amongst others, the methylation status of Lysine residue 4 on Histone 3 (H3K4) represents one of the central dysregulated histone marks in acute myeloid leukemia (AML). Demethylation of H3K4 is mediated by the Lysine (K)-specific demethylase 1A (LSD1/ KDM1A). Remarkably, LSD1 expression is de-regulated in AML and therefore represents an attractive therapeutic target. In fact, pharmacologic LSD1 inhibition was shown to induce AML cell differentiation and apoptosis in combination with all-trans-retinoic acid (ATRA) exposure. Recently, a phase 1 trial with the irreversible LSD1 inhibitor IMG-7289 (IMG) ± ATRA in patients with advanced myeloid malignancies commenced (ClinicalTrials.gov Identifier: NCT02842827). However, the effect of a LSD1-inhibitory therapy on normal human progenitors has not been studied in detail.
Methods: Experiments were performed with mobilized CD34+ cells of deceased multiple myeloma and lymphoma patients. The usage of these cells was approved by the local ethics committee. The following experimental approaches were employed: (1) studying in vitro colony forming unit (CFU) potential of human CD34+ progenitors in the presence of increasing IMG concentrations, (2) investigating the CFU potential in re-plating experiments of primary IMG-7289 exposed CD34+ cell CFU cultures and (3) studying the impact of IMG on in vitro cytokine (SCF, IL-3, IL-6, Flt3L and TPO for 7 days)-driven CD34+ progenitor expansion.
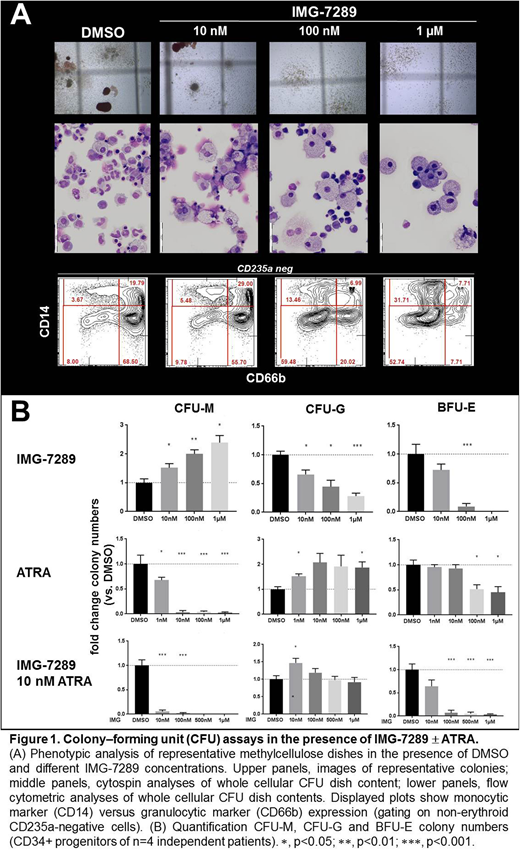
Results: Performing CFU assays in the presence of increasing IMG concentrations revealed a shift from diverse colony types in DMSO control treated CFU assays to increasing phenotypic monocytic (CFU-M) and decreasing granulocytic (CFU-G) and erythroid (BFU-E) colony types (Fig. 1A). Accordingly, increased CFU-M and decreased CFU-G and BFU-E colony numbers were observed (Fig. 1B). In contrast, sole ATRA treatment abrogated CFU-M colony formation, increased CFU-G and slightly decreased BFU-E colony numbers (Fig. 1B). To study the impact of LSD1 inhibition in synergy with ATRA-driven differentiation therapy we treated CFU assays with increasing IMG concentrations in combination with a constant ATRA concentration (10 nM). Strikingly, IMG and ATRA co-exposure severely depleted CFU-M and BFU-E colonies while preserving CFU-G colony formation (Fig.1B). In order to study the impact of IMG on progenitor self-renewal we re-plated IMG-exposed CFU assays in the absence of IMG. In the re-plated dishes we observed a 2.9- and 3.7-fold increase of CFU-G colony numbers compared to the primary CFU-G IMG exposed (100 nM and 1µM, respectively) colony numbers. Finally, we investigated the impact of IMG exposure on in vitro cytokine-driven CD34+ progenitor expansion. In DMSO treated cultures 7 day cytokine incubation led to a 37-fold expansion of CD34+ cell numbers while increasing the total CFU numbers 1.8-fold. Compared to the DMSO control cultures, IMG exposure (100nM and 1µM) boosted CFU-M colony numbers 1.7- and 2.3-fold, respectively. Strikingly, also CFU-G colony numbers were slightly increased by IMG exposure (1.3- and 1.6-fold at 100nM and 1µM, respectively). To investigate how IMG exposure during cytokine-driven CD34+ progenitor expansion alters the transcriptional program of human CD34+ progenitors we analyzed the expression of the hematopoietic regulatory genes GFI1, GFI1B, IRF8 and RARA by real-time PCR. IMG exposure (100 nM) increased the expression of these genes 2.2-, 1.7-, 1.9- and 1.6-fold, respectively. De-repression of GFI1 and GFI1B expression by LSD1 inhibition was expected and the observed upregulation of the monocytic regulator IRF8 is in accordance with the observed increased monocytic colony numbers under LSD1 inhibition. Lastly, RARA upregulation suggested that LSD1 inhibition promotes retinoic acid receptor signaling.
Conclusions: Our findings reveal that pharmacologic LSD1 inhibition increases human monocytic progenitor numbers. Furthermore, in vitro CFU-re-plating and also progenitor expansion experiments point to LSD1 inhibition not depleting granulocytic progenitors. Collectively, our data suggest that expected side effects of a LSD1-targeted therapy such as neutropenia will be reversible.
Rienhoff:Imago BioSciences, Inc.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Dührsen:Janssen: Honoraria; Celgene: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Amgen: Research Funding; Gilead: Consultancy, Honoraria; Roche: Honoraria, Research Funding. Göthert:AOP Orphan Pharmaceuticals: Other: travel support; Proteros Biostructures: Consultancy; Novartis: Honoraria; Pfizer: Consultancy; Incyte: Consultancy, Honoraria, Other: travel support; Bristol-Myers Squibb: Consultancy, Honoraria, Other: travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal